Simulating Lipid Monolayers
- Details
- Last Updated: Wednesday, 10 February 2021 13:20
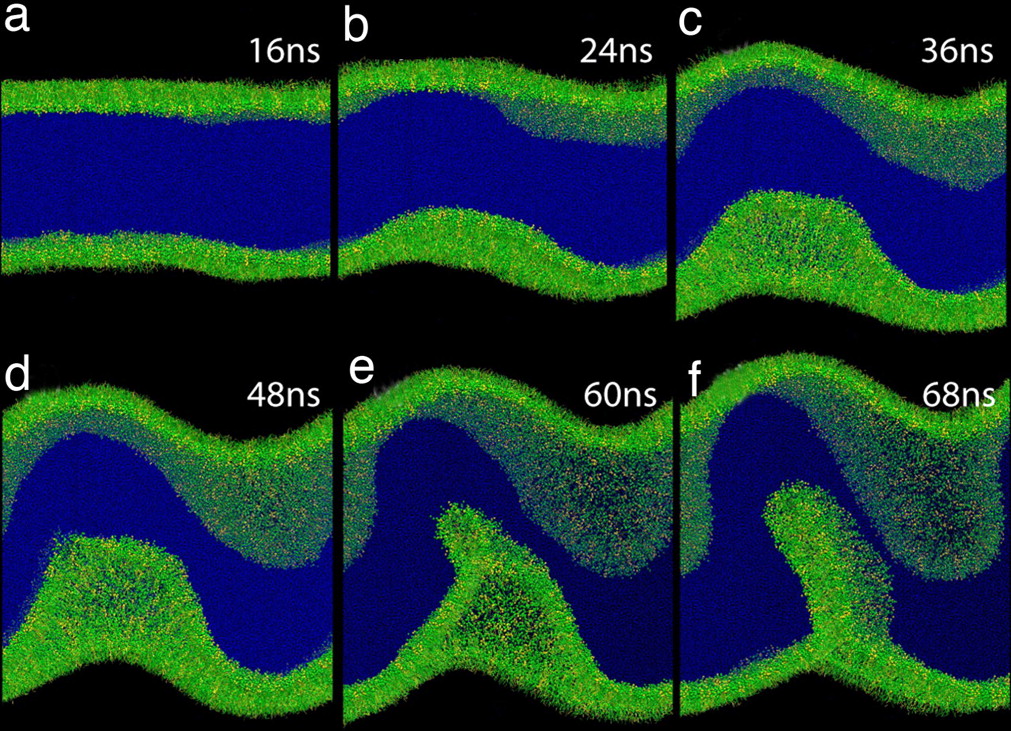
Lipid molecules are insoluble in both polar and apolar media because of their amphipathic nature. At polar–apolar interfaces, they form monomolecular films, monolayers, that reduce the surface tension. The properties of lipid monolayers vary with their surface density. For example, the higher the density, the lower is the resulting surface tension at the interface. At a certain very high surface density, however, a further reduction of the surface tension is not possible: the monolayers become unstable at the interface and collapse. Besides being of fundamental interest for surface science, lipid monolayer collapse is crucial for maintaining low surface tension at the gas-exchange interface in the lungs during breathing.
The Martini force field can be used to study the behavior of lipid monolayers in detail, both for pure lipid systems and in combination with peptides and proteins. Although quantitative reproduction of the pressure-area isotherms at low surface pressure remains challenging [3], the model is well suited to study properties of monolayers in the liquid-expanded and liquid-condensed phase [1,3], and to study for instance the collapse of lipid monolayers at high surface pressure [2] as illustrated by the figure. Mixed monolayers have also been studied, e.g. to model the tear fluid lipid layer [5,6] or look at phase coexistence [7], and to study monolayer collapse in the presence of lung surfactant proteins [8,9]. Compression of bilayers to form tethers has also been addressed with Martini [4,10].
- [1] S. Baoukina, S.J. Marrink, D.P. Tieleman. Lateral pressure profiles in lipid monolayers. Farad. Discuss., 144:393-409, 2010.
- [2] S. Baoukina, L. Monticelli, H.J. Risselada, S.J. Marrink, D.P. Tieleman. The molecular mechanism of lipid monolayer collapse. PNAS, 105:10803-10808, 2008.
- [3] S. Baoukina, L. Monticelli, S.J. Marrink, D.P. Tieleman. The pressure-area isotherm of a lipid monolayer from molecular dynamics simulations. Langmuir, 23:12617-12623, 2007.
- [4] S. Baoukina, S.J. Marrink, D.P. Tieleman. Molecular structure of membrane tethers. Biophys. J., 102:1866-1871, 2012. open access
- [5] P. Kulovesi, J. Telenius, ... I. Vattulainen, J.M. Holopainen. Molecular organization of the tear fluid lipid layer. Biophys. J. 99:2559-2567, 2010.
- [6] J. Telenius, A. Koivuniemi, P. Kulovesi, J.M Holopainen, I. Vattulainen. Role of neutral lipids in tear fluid lipid layer: Coarse-grained simulation study. Just Accepted, 2012. DOI: 10.1021/la304366d
- [7] S. Baoukina, E. Mendez-Villuendas, D.P. Tieleman. Molecular View of Phase Coexistence in Lipid Monolayers. J. Am. Chem. Soc. 134:17543–17553, 2012.
- [8] S. Baoukina, D.P. Tieleman. Lung Surfactant Protein SP-B Promotes Formation of Bilayer Reservoirs from Monolayer and Lipid Transfer between the Interface and Subphase. Biophys. J. 100:1678-1687, 2011.
- [9] S.L. Duncan, R.G. Larson. Folding of lipid monolayers containing lung surfactant proteins SP-B1–25 and SP-C studied via coarse-grained molecular dynamics simulations. BBA-Biomembr 1798:1632-1650, 2010.
- [10] S. Baoukina, H.I. Ingólfsson, S.J. Marrink, D.P. Tieleman. Curvature‐Induced Sorting of Lipids in Plasma Membrane Tethers. Advanced Theory Simul., 1:1800034, 2018. doi:10.1002/adts.201800034


























































































































































